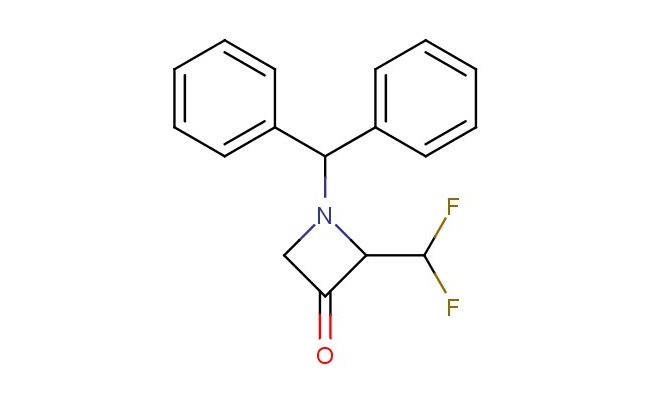
1-benzhydryl-2-(difluoromethyl)azetidin-3-one
$400.00
CAS No.: 2231676-63-2
Catalog No.: 200391
Purity: 95%
MF: C17H15F2NO
MW: 287.309
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)(C1=CC=CC=C1)N1C(C(C1)=O)C(F)F
Catalog No.: 200391
Purity: 95%
MF: C17H15F2NO
MW: 287.309
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)(C1=CC=CC=C1)N1C(C(C1)=O)C(F)F
For R&D use only. Not for human or animal use.
CAS NO.: 2231676-63-2;1-benzhydryl-2-(difluoromethyl)azetidin-3-one. PROPERTIES: This fluorinated beta-lactam presents as white crystalline solid with molecular weight approximately 267.3 g/mol, combining azetidinone ring strain with benzhydryl and difluoromethyl groups. The 1-benzhydryl-2-(difluoromethyl)azetidin-3-one exhibits limited aqueous solubility but good dissolution in DMSO and NMP. Stability testing reveals vulnerability to acid-catalyzed lactam ring-opening and base-promoted benzhydryl deprotection, necessitating storage at 2-8 degree Celsius in borosilicate glass. Safety protocols require using powder hoods with HEPA filtration and wearing aluminized suits during bulk handling. Skin contact may cause protein denaturation requiring immediate washing with mild acids. Inhalation may induce chemoreceptor trigger zone stimulation; treatment includes antiemetic administration. Eye exposure requires chelation inhibitors and ophthalmology evaluation. Spills should be contained with alcohol-resistant foams. APPLICATIONS: The 1-benzhydryl-2-(difluoromethyl)azetidin-3-one functions as a key intermediate in carbapenem antibiotic synthesis through ring expansion reactions. Its difluoromethyl group provides enhanced beta-lactamase resistance compared to monofluorinated analogs. The compound serves as a building block for creating non-classical beta-lactam antibiotics with broader spectrum activity. Additionally, it undergoes Staudinger reaction for creating azetidine-based phosphonium salts. Research teams utilize it as a starting material for creating beta-lactam-based organocatalysts with defined stereochemistry. In materials science, its strained beta-lactam ring enables creation of molecular containers with selective guest binding.
Reviews
Write Your Own Review