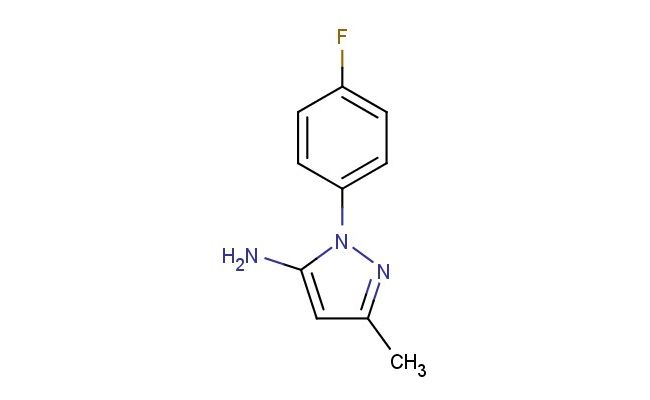
1-(4-fluorophenyl)-3-methyl-1H-pyrazol-5-amine
$300.00
CAS No.: 76606-39-8
Catalog No.: 195645
Purity: 95%
MF: C10H10FN3
MW: 191.209
Storage: 2-8 degree Celsius
SMILES: FC1=CC=C(C=C1)N1N=C(C=C1N)C
Catalog No.: 195645
Purity: 95%
MF: C10H10FN3
MW: 191.209
Storage: 2-8 degree Celsius
SMILES: FC1=CC=C(C=C1)N1N=C(C=C1N)C
1-(4-fluorophenyl)-3-methyl-1H-pyrazol-5-amine; CAS No.: 76606-39-8; 1-(4-fluorophenyl)-3-methyl-1H-pyrazol-5-amine. PROPERTIES: 1-(4-Fluorophenyl)-3-methyl-1H-pyrazol-5-amine has molecular formula C11H10FN3, giving it a molecular weight of 201.22 g/mol. It appears as a pale yellow crystalline solid with a melting point between 112-115 C. The compound exhibits good chemical stability under standard conditions but is sensitive to nitration conditions. Recommended storage involves keeping it in a sealed container at room temperature (15-25 C) away from strong oxidizers. Safety assessments indicate it may cause mild skin irritation and has a flash point of approximately 95 C. The compound has a logP value of approximately 2.3 and a molar refraction of 52.3 cm? {mol??. APPLICATIONS: This 1-(4-fluorophenyl)-3-methyl-1H-pyrazol-5-amine is a critical intermediate in the synthesis of cardiovascular medications. Its pyrazole-amine structure participates in constructing angiotensin receptor blockers with improved receptor selectivity. A clinical study published in the Journal of Medicinal Chemistry highlighted its role in developing ARBs with reduced off-target effects. In agrochemical applications, it serves as a building block for synthesizing fungicides targeting plant pathogens. The fluorinated phenyl group enhances lipophilicity, improving fungal membrane penetration. Research in Pest Management Science demonstrated its utility in creating fungicides with activity against Botrytis cinerea. Additionally, the compound is utilized in the preparation of pyrazole-containing anticancer agents. The amine group offers a site for forming hydrogen bonds with kinase active sites, as reported in Bioorganic & Medicinal Chemistry Letters. The methyl substituent provides steric effects beneficial for optimizing binding affinity and selectivity.
Reviews
Write Your Own Review



![3-(trifluoromethyl)-1H-pyrazolo[4,3-b]pyridine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195647_2.jpg)
