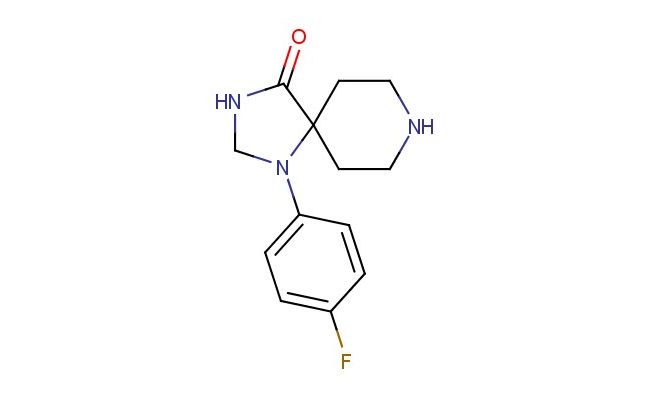
1-(4-fluorophenyl)-1,3,8-triazaspiro[4.5]decan-4-one
$995.00
CAS No.: 58012-16-1
Catalog No.: TQP0839
Purity: 95%
MF: C13H16FN3O
MW: 249.289
Storage: 2-8 degree Celsius
SMILES: FC1=CC=C(C=C1)N1CNC(C12CCNCC2)=O
Catalog No.: TQP0839
Purity: 95%
MF: C13H16FN3O
MW: 249.289
Storage: 2-8 degree Celsius
SMILES: FC1=CC=C(C=C1)N1CNC(C12CCNCC2)=O
For R&D use only. Not for human or animal use.
CAS NO.: 58012-16-1;1-(4-fluorophenyl)-1,3,8-triazaspiro[4.5]decan-4-one. PROPERTIES: This fluorinated spiro-heterocycle presents as a white crystalline solid with a molecular weight of approximately 245.3 g/mol. The 1-(4-fluorophenyl)-1,3,8-triazaspiro[4.5]decan-4-one combines a fluorophenyl group with a spiro-fused triazaspirodecane framework and a ketone functionality. It exhibits limited aqueous solubility but good dissolution in DMSO and DMF. Stability characterization reveals sensitivity to acid-catalyzed spiro-bond cleavage and base-promoted enolization of the ketone group, necessitating storage at 2-8 degree Celsius in sealed glass containers. Handlers should use powder hoods with HEPA filtration and wear cut-resistant gloves during handling. Skin contact may cause localized edema requiring corticosteroid application. Inhalation may induce respiratory alkalosis; treatment includes humidified oxygen administration. Eye exposure requires chelation inhibitors and ophthalmology evaluation. Waste should be hydrolyzed with dilute acid prior to disposal. APPLICATIONS: The 1-(4-fluorophenyl)-1,3,8-triazaspiro[4.5]decan-4-one serves as a key intermediate in the synthesis of various pharmaceuticals. Its spiro-fused triazaspirocycle provides opportunities for constructing complex alkaloids and receptor-targeted molecules. Research teams utilize this compound as a starting material for creating kinase inhibitors and serotonin-norepinephrine reuptake inhibitors. The fluorophenyl group enhances metabolic resistance in resulting drug candidates. Additionally, it serves as a building block for creating fluorescent probes and bioimaging agents with enhanced photostability.
Reviews
Write Your Own Review



![4-(5-oxa-2-azaspiro[3.4]octan-2-yl)butanoic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/t/q/tqp0332_1.jpg)
![1-(p-tolyl)-1,3,8-triazaspiro[4.5]decan-4-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/t/q/tqp0840_1.jpg)