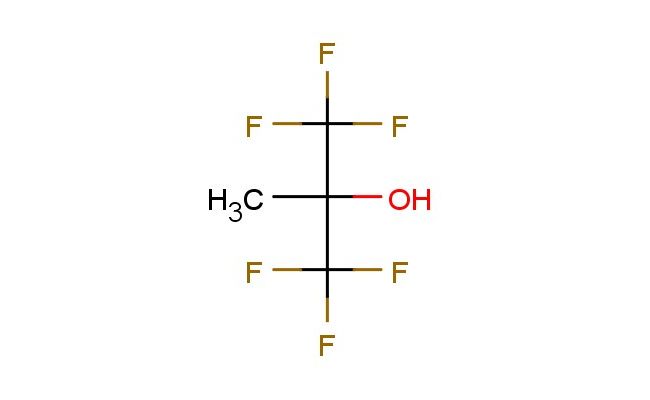
1,1,1,3,3,3-hexafluoro-2-methylpropan-2-ol
$200.00
CAS No.: 1515-14-6
Catalog No.: 200526
Purity: 95%
MF: C4H4F6O
MW: 182.063
Storage: 2-8 degree Celsius
SMILES: FC(C(C(F)(F)F)(O)C)(F)F
Catalog No.: 200526
Purity: 95%
MF: C4H4F6O
MW: 182.063
Storage: 2-8 degree Celsius
SMILES: FC(C(C(F)(F)F)(O)C)(F)F
For R&D use only. Not for human or animal use.
CAS NO.: 1515-14-6;1,1,1,3,3,3-hexafluoro-2-methylpropan-2-ol. PROPERTIES: This highly fluorinated alcohol presents as a colorless liquid with a molecular weight of approximately 182.08 g/mol. The 1,1,1,3,3,3-hexafluoro-2-methylpropan-2-ol combines a central tertiary alcohol with six fluorine substituents, exhibiting exceptional solubility in polar aprotic solvents and limited water miscibility. Stability characterization reveals extraordinary chemical resistance to acids and bases but vulnerability to strong reducing agents. The compound requires storage at 2-8 degree Celsius in PTFE-lined containers to prevent surface adsorption. Safety measures include using polyethylene transfer devices and maintaining handling temperatures below 25 C. Skin contact may cause defatting dermatitis requiring emollient application. Inhalation of vapors may induce hypocalcemia; treatment includes calcium gluconate administration. Eye exposure requires immediate rinsing with saline solution and possible corticosteroid application. Waste should be incinerated at >1200 C to prevent perfluorocarbon formation. APPLICATIONS: The 1,1,1,3,3,3-hexafluoro-2-methylpropan-2-ol serves as a specialty solvent in the preparation of fluorinated pharmaceuticals and agrochemicals (excluding agricultural applications). Its unique polarity enables extraction of labile biomolecules in proteomics workflows. The compound functions as a key intermediate in the synthesis of liquid crystal monomers for display technologies. Additionally, it undergoes triflation to create perfluoroalkyl intermediates for Orgel cyclization reactions. Research teams utilize it as a starting material for creating fluorinated surfactants with enhanced surface activity. In materials science, its trifluoromethyl groups enable creation of superhydrophobic coatings for aerospace applications.
Reviews
Write Your Own Review